UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
____________________________________________________________________________________________
FORM 8-K
____________________________________________________________________________________________
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): November 15, 2017
____________________________________________________________________________________________

OPTINOSE, INC.
(Exact Name of Registrant as Specified in its Charter)
____________________________________________________________________________________________
DELAWARE | 001-38241 | 42-1771610 |
(State or Other Jurisdiction of Incorporation or Organization) | (Commission File No.) | (I.R.S. Employer Identification No.) |
1020 Stony Hill Road, Suite 300
Yardley, Pennsylvania 19067
(Address of principal executive offices and zip code)
(267) 364-3500
(Registrant’s telephone number, including area code)
(Former name or former address, if changed from last report)
____________________________________________________________________________________________
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
q | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
q | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
q | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
q | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-14(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter). | |
ý | Emerging growth company |
ý | If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. |
Item 7.01 Regulation FD Disclosure.
Corporate Presentation
On November 15, 2017, OptiNose, Inc. posted an updated Corporate Presentation on its website www.optinose.com. A copy of the presentation is furnished hereto as Exhibit 99.1 and is incorporated by reference herein.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
Exhibit No. | Description | |
99.1 | Corporate Presentation dated November 15, 2017 | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, hereunto duly authorized.
OptiNose, Inc. | ||
By: /s/ Keith A. Goldan | ||
Keith A. Goldan | ||
Chief Financial Officer | ||
Date: November 15, 2017
EXHIBIT INDEX
Exhibit No. | Description | |
99.1 | ||

Building a Leading Specialty Biopharma
Company in ENT / Allergy
Company Presentat ion
1 5 N o v e m b e r 2 0 1 7

2
Forward Looking Statements
This presentation and our accompanying remarks contain “forward-looking statements” within the meaning
of the U.S. Private Securities Litigation Reform Act of 1995. All statements that are not historical facts are
hereby identified as forward-looking statements for this purpose and include, among others, statements
relating to: the launch of XHANCE in the second quarter of 2018; initiation and timing of clinical trials for
chronic sinusitis; market opportunities; commercial strategies; potential advantages of XHANCE and our
product candidates; and other statements regarding our future operations, financial performance, prospects,
intentions, objectives and other future events.
Forward-looking statements are based upon management’s current expectations and assumptions and are
subject to a number of risks, uncertainties and other factors that could cause actual results and events to
differ materially and adversely from those indicated by such forward-looking statements including, among
others: our ability to establish supply chain, commercial and other capabilities to launch XHANCE; physician
and patient acceptance of XHANCE; our ability to obtain adequate third-party reimbursement for XHANCE;
uncertainties relating to the initiation, completion and results of pre-clinical and clinical trials; market
opportunities for XHANCE may be smaller than we believe; and the risks, uncertainties and other factors
discussed in the “Risk Factors” section and elsewhere in our filings with the Securities and Exchange
Commission – which are available at http://www.sec.gov.
As a result, you are cautioned not to place undue reliance on any forward-looking statements. Any forward-
looking statements made in this presentation speak only as of the date of this presentation, and we
undertake no obligation to update such forward-looking statements, whether as a result of new information,
future developments or otherwise.

3
Emerging Growth Company with Approved Products
BUILDING A LEADING ENT / ALLERGY SPECIALTY COMPANY
• 3.5 million CRS patients (1.2M with nasal polyps) being treated by 15,000 physicians
• Limited competition anticipated from any pharma companies at launch
XHANCE Represents a Significant Opportunity in Attractive ENT/Allergy Market
• Potential to be first product approved for chronic sinusitis indication—trials planned to start 2H2018
• Expected to support expansion to primary care physicians treating an additional 6.25M patients
“Pipeline Within a Product” Creates Substantial Near-Term Value
• Product candidates have been identified that could be developed using EDS platform for ENT/allergy market
• External pipeline products also identified for potential partnering or acquisition in ENT/allergy
Additional Pipeline Focused on Products for ENT/Allergy to Leverage Infrastructure/Expertise
• Several candidates (eg, Narcolepsy, Prader-Willi) take advantage of “nose-to-brain” delivery concept
• Plan development through proof-of-concept and then to seek partnerships for further growth
Create Additional Value by Early Development of Additional EDS Platform Products

4
Attractive Market Opportunity in Chronic Rhinosinusitis (CRS)
Potential to Become Part of the Standard of Care
Potential Favorable Market Access due to Pricing Strategy & Pharmacoeconomics
Concentrated Target Market Activated Through Specialty Sales Force
Patent Portfolio and Regulatory Barriers Support Potential Long-Term Opportunity
Proven Management Team with Big Pharma and Emerging Healthcare Experience
Excellent Potential in a Large Market with Unmet Need

5
Experienced Leadership Team
Peter Miller
Chief Executive Officer and Director
Ramy Mahmoud, MD, MPH, FACP
President & Chief Operating Officer
Tom Gibbs
Chief Commercial Officer
Keith Goldan
Chief Financial Officer

6
Mild Moderate Severe
• Highly prevalent in the
United States (~65M)
• Largely OTC / generic
market
• Well-treated using INS
Allergic Rhinitis
* Based on US Adult Population Survey (n=10,336).
• ~30M US adults suffer from
CRS—up to 10M with Nasal
Polyps
• 9.75M patients actively seeking
physician care annually
• 7M patients have had surgery
(frequently non-curative)
• CRS is divided into two major sub
groups: CRS with and without
Nasal Polyps
Chronic
Rhinosinusitis (CRS)
Allergic
Rhinitis
65M
Perennial
Rhinitis
18M
Chronic
Rhinosinusitis
w/o NP
20M
w/ NP
10M
Large CRS Population with Severe Symptoms

7
• A diagnosis characterized by
chronic inflammation
• Persistent inflammation causes pain and
obstruction deep in the nasal passages and
openings to the sinuses
• Primary defining symptoms include congestion,
facial pain/pressure, rhinorrhea and loss of
smell/taste
• Acute flares are frequent complications of CRS
CRS with or without Polyps
THE ROOT ISSUE IS INFLAMMATION (NOT INFECTION)

8
CRS Patients Suffer High Disease Burden
Source: Naïve patient survey, Physician survey.
Patients Frequently Endure Severe
Symptoms Throughout the Year
• Disease persists for many years
• Significant Quality of life impact (comparable to
CHF, COPD, Angina)
Additional Symptoms are Common and can
be Serious
• Including chronic sleep disruption, headaches,
fatigue and mood disorders

9
High Cost, Typically Not Curative
Sinus Surgery
~80% of patients continue to have symptoms
after surgery
Continuing Nasal Steroid
use after surgery is typical
$8,500–$16,000 per procedure, and repeat
surgery is not uncommon
Limited Efficacy
Medical Management
Saline nasal spray, irrigations, neti pots, nebulizers,
conventional nasal steroids, oral steroids
~80% of patients are frustrated with
lack of symptom relief
~75% of physicians believe nasal spray steroids do
not work well because they don’t sufficiently
reach site of inflammation
Existing Treatments are Sub-Optimal
LIMITED EFFICACY, COSTLY, DIFFICULT, PAINFUL, FREQUENTLY NOT CURATIVE
Source: Recurrence of Nasal Polyps After Functional Endoscopic Sinus Surgery Abstract.

10
Problem:
Nasal sprays and aerosols do not effectively place drug
high and deep in the nasal passages
Solution:
Unique new concept for delivery gets medicine where it
needs to be to work
Drug
released
into
airflow
Pressure
balances
Soft palate
closes
Blow into
device
Press bottle up to open device valve
and actuate
• Proprietary exhalation delivery systems (EDS) have a mouthpiece
and sealing nosepiece
• Exhaled air passes through the EDS and drug is added
• Delivery takes advantage of natural behaviors of the upper airways
Exhaled breath naturally seals the soft palate then flows in one side and
then out the opposite side of the nose
• Simple, quick use with limited coordination requirements
• “Positive pressure” delivery expands narrow passages
Helps “float” drug behind barriers to broadly fill one side of the nasal cavity
• Drug is deposited high and deep in the nasal passages
Breakthrough Approach to Nasal Delivery
SOLVES A KNOWN MEDICAL PROBLEM WITH A UNIQUE NEW APPROACH

11
Optinose EDS
Traditional Spray
Intranasal steroids are well-tolerated TOPICAL TREATMENTS
and only work where they are delivered
Tight spaces in the
region where
sinuses normally
open and drain
Optinose EDS Delivers Drug High and Deep in the Nose
KEY TO TREATING CS (with or without polyps) IS REACHING TARGET REGIONS

12
Trial Type N Sites
NAVIGATE I
Phase 3
pivotal
323 54
NAVIGATE II
Phase 3
Pivotal
323 38
EXHANCE-3
Phase 3
open-label
3 month
700 38
EXHANCE-12
Phase 3
open-label
12 month
223 21
Study 1102
Phase 1
bioavailability
112 2
Global Clinical Program
Significant benefit on all four defining
symptoms of CS
Magnitude of relief comparable to surgery
“Medical” polyp elimination in some patients
Similar improvements in patients with and without
nasal polyps
Reduction in eligibility for surgery
Approximately 70% of patients reported being “much”
or “very much” improved
Key Highlights
Differentiated Clinical Profile
1,500+ Patients
792 CS w/o polyps
780 CS w/ polyps

13
* Not currently in guidelines.
^ Currently marketed for other indications and in development for nasal polyps indication.
Potential to Become Part of Standard of Care
ADAPTED FROM INTERNATIONAL CONSENSUS STATEMENT ON ALLERGY
AND RHINOLOGY (ICAR)
*
After Failure of Current
Medical Management
INVASIVE, EXPENSIVE,
INCREASED RISK
• Endoscopic Sinus
Surgery (ESS)
• Emerging Monoclonal
Antibodies*^
Potential payer exposure
Recommended
TOPICAL and INEXPENSIVE
but OFTEN INSUFFICIENT
• Intranasal Steroids
(INS)
• Saline irrigation

14
WAVE 1
Enter High-Density
ENT/Allergy Specialty Market
• 15,000 Physician Targets
• Ramping up to 150 Sales Reps
• Limited Competition
• Promotion of Nasal Polyp Indication
~3.5M Patients
(1.2M with Nasal Polyps)
WAVE 2
Facilitate Broader Adoption
in Primary Care
• Future Chronic Sinusitis indication
facilitates broadening target market
• Additional 50,000 Primary Care Targets
• May pursue co-promote partner
~6.25M Patients
WAVE 3
Activate Patient Demand
• Significant Direct to Patient Opportunity
• Lapsed Users still suffering
• Symptomatic nature allows patients to
self ID
~20M Patients
Launch
Expected
in 2Q
2018
Commercialization Strategy to Build XHANCE into a Leading Product

15
96.1%
0
5
10
15
20
25
30
35
40
45
Database
Population
% of Population
with no CRS Claim
% of Population
with CRS Claim
P
at
ie
n
t Cla
im
s Da
ta
b
as
e
41M
3.9%
30M US Adults Suffer from Chronic Rhinosinusitis
~9.75M PATIENTS CURRENTLY UNDER ACTIVE CARE OF A PHYSICIAN FOR CRS
Approximately 3.9% of patients in claims database
have a code for CRS (2010–2012) ~9.75M CRS Patients being treated in physician offices
US Adult Population
Prevalence of
CRS Claims
ICD9 Code: 471.x and
473.x
250 Million
3.9%
9.75M
CRS Patients being Treated in
Physician Office
Patients Treated
CRS Unique Patient Claims
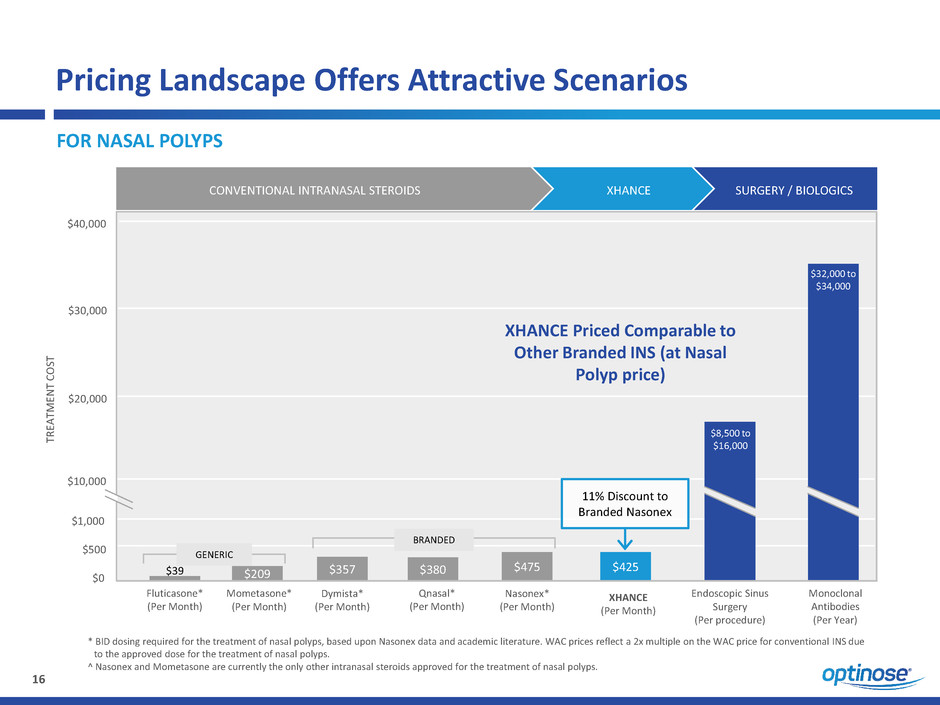
16
* BID dosing required for the treatment of nasal polyps, based upon Nasonex data and academic literature. WAC prices reflect a 2x multiple on the WAC price for conventional INS due
to the approved dose for the treatment of nasal polyps.
^ Nasonex and Mometasone are currently the only other intranasal steroids approved for the treatment of nasal polyps.
Pricing Landscape Offers Attractive Scenarios
FOR NASAL POLYPS
$0
$500
$1,000
$10,000
$20,000
$30,000
$40,000
Mometasone*
(Per Month)
Fluticasone*
(Per Month)
Nasonex*
(Per Month)
Qnasal*
(Per Month)
Dymista*
(Per Month)
Endoscopic Sinus
Surgery
(Per procedure)
Monoclonal
Antibodies
(Per Year)
$8,500 to
$16,000
$32,000 to
$34,000
$475
CONVENTIONAL INTRANASAL STEROIDS XHANCE
$425
XHANCE
(Per Month)
SURGERY / BIOLOGICS
$39 $380$357$209
TR
EA
TM
EN
T
C
O
ST
XHANCE Priced Comparable to
Other Branded INS (at Nasal
Polyp price)
11% Discount to
Branded Nasonex
GENERIC
BRANDED

17
PREVALENCE
OVERALL TREATED PATIENTS
TREATED PATIENTS
IN SEGMENT
TOTAL ANNUAL US MARKET
OPPORTUNITY
SEGMENT ANNUAL MARKET
OPPORTUNITY
INCREMENTAL PCP OPPORTUNITY
(1) Target market represents ~10,000 ENT and allergy specialists and ~5,000 high-decile INS prescribing primary care physicians.
(2) Based on our internal estimates.
SPECIALTY MARKET OPPORTUNITY
~3.5 Million
CRS patients currently treated in ENT /
Allergy specialty offices annually
~6.25 Million
CRS patients currently treated by primary
care physicians annually
KEY DRIVER
$3.4B Market Opportunity Within Specialty
(NP and CS Indications)
TOTAL MARKET OPPORTUNITY OF >$9.5B (NP and CS Indications)
30 Million Chronic Rhinosinusitis Sufferers in US
(7 Million Have Had Surgery)
9.75 Million
CRS patients currently in physician
offices being treated annually
ENT & Allergy Specialty Segment(1)
>$3.4B(2)
Primary Care Segment
>$6.0B(2)
Total Specialty and Primary Care
>$9.5B(2)

18
0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
Allergists ENTs PCPs
Lik
el
ih
o
o
d
t
o
Pr
es
cr
ib
e
N=200 N=202 N=302
Percent of HCPs stating they will “definitely” or “probably” prescribe XHANCE
Differentiated Physician Reaction to Profile
SUGGESTS HIGH LAUNCH INTEREST AND STANDARD OF CARE POTENTIAL
Physicians of all Specialties Express High Interest in
Prescribing
Physician Stated Interest
“ ”…There is a real need in the medical community to be able to deliver intranasal steroids higher and deeper in the nasal
cavity…
Allergist / Immunologist
“ ”…If the Optinose Device is approved, I will definitely use it in my practice; no question….
Otolaryngologist
“ ”… The Optinose Device will be a game changer… and will really improve our ability to care for patients….
Director, Division of Rhinology

19
Favorable Pharmacoeconomic Profile Offers Promising
Market Access Dynamics
RESEARCH* INCLUDING 25 PAYORS REPRESENTING OVER 150M LIVES
PRODUCT
Payors grasp the underlying science/technology of
XHANCE
PRICE
Need to Price “reasonably”
CONTROL
Payors “do not” / “do not want” to manage actively
MARKET ACCESS
Most commercial lives will have acceptable access if
there is comparable pricing to branded INS and
utilization is focused on CRS
*Q4 2015. Payors Assumed Utilization Both Within NP and More Broadly in CRS.
Potential Commercial Coverage Reported
by Surveyed Payors
Percentage of Commercial Lives
27%
48%
7%
15%
Formulary Status
Not Covered
Hard PA
Soft PA
Single Step Edit
Non Preferred
(No Step)
Across the top 12 payer accounts, for 80% of the total
covered lives, payers currently cover INS through open
access or step edits

20
Efficient, Specialty-Focused, Go-to-Market
Commercialization Model to Launch XHANCE
XHANCE Territories
(assuming full deployment)
Approximately 75 designated
territory managers at launch
growing to 150 based on
expansion of market access
Specialties:
ENT, allergists and high-decile,
INS-prescribing primary care
physicians
~15,000
Targets

21
Multi-Channel Approach to Drive Rapid
Trial and Adoption
Pre-PDUFA Post Approval/Pre-Launch Launch
Jul Aug Sep Oct Nov Dec Jan Feb Mar Apr May Jun Jul Aug Sep
DIGITAL, SOCIAL
AND NPP
CLINICAL NURSE
EDUCATORS
OPTINOSE SALES
FORCE
75 Sales Representatives Deployed Based Upon Access
Planning for ~200,000 Samples
Planning for ~700 Speaker Programs
Corporate / EDS Campaign
10,547 Targets (6,292 Offices)
Reach Target 90% | Frequency Target: 4

22
Optinose EDS Finally Enables Delivery of Drug to Target Sites
High/Deep in the Nose: Differentiated Clinical Profile
Brand
Differentiation
Unmet Need
• Patients and physicians cite limited efficacy as the most
important unmet need for treating CS with or without Nasal
Polyps
Unsatisfied / engaged patient
• Target patient type is patients who have already tried and
failed on a traditional intranasal steroid
Innovative delivery system
Delivers excellent clinical benefit
• Significant improvement on all 4 defining symptoms of CS
• “Medical” polyp elimination in some patients
• Magnitude of efficacy similar to surgery (SNOT-22)
• Reduction in surgical eligibility

23
PBM Access Planning Scorecard
PBM Q3 2017 Q4 2017 through Q1 2018
Lives: 28.0M
Lives: 36.0M
Lives: 11.0M
Lives: 1.0M
Lives: 9.0M
Task Completed
Complete introductory presentation
Complete clinical presentation
Submit term sheet
Potential formulary approval
Key

24
Attractive Market Opportunity in Chronic Rhinosinusitis (CRS)
Potential to Become Part of the Standard of Care
Potential Favorable Market Access due to Pricing Strategy & Pharmacoeconomics
Concentrated Target Market Activated Through Specialty Sales Force
Patent Portfolio and Regulatory Barriers Support Potential Long-Term Opportunity
Proven Management Team with Big Pharma and Emerging Healthcare Experience
Excellent Potential in a Large Market with Unmet Need
